This is the presentation about Thoracic Outlet Syndrome of Alfredo Dente
Blog
Lorenzo Jovanotti about Alfredo Dente

It’s the right recognition for my miracles team.” (Blu Media)
2012 “ORA tour singer Lorenzo Jovanotti will rely again on the assistance of his long term therapist Fabrizio Borra and in his absence Alfredo Dente will replace him” Fisiology Forlì
Casey Stoner and his physiotherapist Alfredo Dente, Ducati MotoGP Team
MotoGP Laguna Seca 2009, Casey Stoner – Ducati MotoGP Team – 4th
“It has been a weekend to forget. I can’t be happy with fourth place even if it has limited the damage to my championship chances. Today I didn’t feel as bad at the end of the race as I have done in the last two but I’m still not right. I found it really difficult to keep my concentration over the last few laps and I was in a bit of a daze by the end. My left arm was feeling really tired by the end because of the fact there are so many left-handers here and my left wrist still doesn’t have the full range of movement after my operation in the winter. I’m really disappointed because without all of these problems we could have done so much better. I want to thank the team for giving me a great bike and also Doctor Claudio Macchiagodena and our physiotherapist Freddie (Dente) for all their help. Now we’ll focus on trying to find out the root of the problem because racing in this condition is not much fun.” (motorcycle-usa.com)
MotoGP Catalunya 2009, Monday Test Info
“This morning, when I woke up, I had some cramp in my legs and a backache so I did some physiotherapy con Freddy (Dente, the team’s physiotherapist) and then got on the bike. I was not in perfect shape and I couldn’t make more than a couple of runs at a time. Despite this, we managed to test what we needed to test, the set-up of the bike, the carbon fork and a new rear shock. I’m fairly happy because the test has confirmed what we thought and I think we are improving bit by bit. We are still lacking some rear grip but we we’ve found a few ways to improve the bike and I am optimistic. Now I’m looking forward to resting and to getting back to 100% fitness in time for Assen.” (superbikeplanet.com)
“Gracias al equipo por su trabajo y a mi fisioterapeuta, Freddie Dente, por ayudarme a estar en la carrera con una fractura en la muñeca.”
“Merci à l’équipe pour leur travail et à mon physiothérapeute Freddie Dente, pour m’aider à gérer la course avec une fracture au poignet.“
Leonardo Araújo and Alfredo Dente selfie
Look who has popped into my studio today….thank you @LeonardoNDA for this selfie
Classification of connective tissue
According to Gray’s Anatomy (Williams 1995, page 75), structural connective tissues are conveniently divided into:
• General or ordinary types, which is widely distributed,
• Special skeletal types, namely cartilage and bone, and
• Haemolymphoid tissue and their precursors
The following paragraphs will describe the general connective tissue, because the description of the other types of connective tissue is out of the purpose of this text.
General connective tissue
Connective tissue is formed of cells and extracellular matrix (fig.1). The matrix in turn is composed of fibres and ground substance. The ground substance is a jelly, aqueous environment. This environment contains collagen fibrils and is controlled by specifically attached proteoglycan molecules. Haemal tissue of the bone marrow or lymphoid tissue provides connective tissue with a number of cell types. The cells reach the connective tissues by the circulating blood and migrate into them through their endothelial walls (Williams 1995).

According to Gray’s Anatomy (Williams 1995, page 75-80), cells of general connective tissue can be separated into:
1. The resident cell population (fibroblasts, adipocytes, persistent mesenchymal stem cells, etc)
2. The immigrant population. They are wandering cells with various defensive functions (macrophages, lymphocytes, mast cells, neurotrophils and eosinophils).
1. The resident cell population.
Fibroblasts are usually the most numerous cells. They synthesize and secrete most of proteins of the extracellular matrix of connective tissue. They transform into fibrocytes when they become old and inactive.
Fibroblasts lay down collagen and elastin fibres and adhere to them. During wound repair, they are predominantly active by multiplying and laying down a fibrous matrix. Various factors such as steroid hormone levels, dietary content and mechanical stress affect their activity. Vitamin C deficiency leads to an impairment of collagen formation.
Adipocytes (lipocytes, fat cells) exist singly or in groups in the meshes of many but not all connective tissues. They are specially numerous in adipose tissue.
Pigment cells occur in the dermis of skin and in the iris and choroid of eye. Their function is generally to prevent light from reaching adjacent cells.
2. The immigrant cell population
Macrophages (histocytes) are also numerous in connective tissues, where they may be either attached to matrix fibres (stationary or fixed macrophages) or they may be motile (nomadic macrophages). These cells are important phagocytes, which are particularly active during inflammation.
Lymphocytes. These cells are typically present in small numbers, but are numerous in pathological states, migrating from adjacent lymphoid tissue or from circulation.
Mast cells (histaminocytes) are important defensive cells, which occur particularly in loose connective tissues and often in the fibrous capsules of some organs such as liver. They are associated with the inflammation. They migrate to the injured tissue where they may be disrupted to release their contents such as histamin, heparine. The signal for this migration is the direct mechanical or chemical trauma or the contact with specific antigens to which the body has previously been exposed.
Neutrophil and Eosinophil leucocytes. They frequently present in small numbers but when the tissues are infected, they migrate to them in increased numbers.
Extracellular matrix
The presentation of the ECM will concentrate on those elements that have prominent mechanical functions. In this sense, the ECM consists of 3 types of macromolecules – fibres (collagen and elastin), proteoglycans (PGs) and glycoproteins. Each of these macromolecules is synthesized and maintained by cells, which are specific to the tissue type (Culav et al 1999).
1.Fibres.
The two most important fibrous components of the ECM are a) collagen and b) elastin, which are insoluble macromolecular proteins. The most prominent feature of collagen is their ability to resist tensile loads. Generally, under tension, they show minimal elongation (less than 10%). This is not the true elongation of the individual fibres. It is the result of the straightening of fibres that are crowded in various 3-dimensional arrangements. Conversely, when a force stretches elastic fibres, they may increase their length by 150%, and after the force has been removed, they return to their previous configuration (Culav et al 1999).
a) Collagen. Collagen is the most abundant protein in the human body, accounting for nearly 30% of all protein (Williams 1995). Nineteen different types of collagen fibres have been found by 1999, all with individual characteristics that serve specific functions in a variety of tissues (Liu et al 1995, Mayne 1997, Culav et al 1999).
The “collagen family” is divided into four subclasses (Burgeson et al 1992, Williams et al 1997):
• Fibrillar collagens (type I, II, III, V, XI)
• Basement membrane-associated collagen (type IV, VII)
• Fibre-Associated (type IX, XII)
• Short-Chain (type VIII, X)
The following paragraphs will describe the fibrillar collagen types I, II and III, which are among the most abundant proteins in the body and are the most relevant to physical therapists.
The fibrillar collagens are estimated to constitute over 70% of the total collagen found in the body (Culav et al 1999). Collagen type I has the broadest distribution (Liu et al 1995, Hukins 1990).
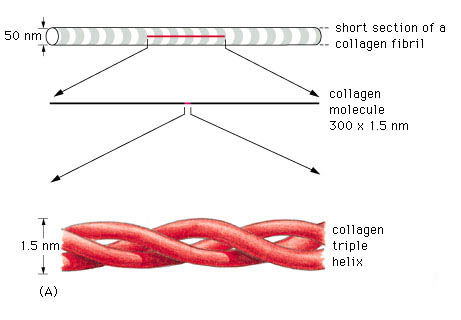
A triple helix is the general structural feature that identifies the fibrillar collagens (fig.2). The diameter and length of the fibrillar collagens vary. Their surfaces have characteristic striations formed by gaps between the ends of the molecules (fig.3) (Williams 1995).
Type I collagen account for 60% to 90% of the dry weight of skin, ligaments, tendon and bone (demineralised) (Mayne 1997, Williams et al 1997). It is also found in many thin tissues including the lungs and dentin and the sclera of the eyes. In addition, it is the major constituent of mature scars (table 1) (Williams et al 1997). Furthermore, it is found in annulus fibrosus of the intervertebral disc, fibrous cartilage and fascia (Liu et al 1995, Culav et al 1999).
Type II collagen accounts for more than half the dry weight of hyaline and elastic cartilage, and it is found in the nucleus polposus and in the vitreous gel of eye (table 1). It is also transiently present in many tissues during embryonic development (Williams et al 1997, Liu et al 1995, Culav et al 1999).
| Collagen type | Tissue distribution | Ultra-structure | Site of synthesis | Function |
| Type I | Dermis, bone, tendon, teeth, fascia, sclera, organ capsules, fibrous cartilage | Densely packed thick fibrils with marked variation in diameter | Fibroblast, osteoblast, odondoblast | Resistance to tension |
| Type II | Hyaline and elastic cartilage, intervertebral disc, vitreous | No fibres; very thin fibrils embedded in abundant ground substance | Chondroblast | Resistance to tension |
| Type III | Bone, skin, smooth muscle, arteries, uterus, liver, spleen, kidney, lung tendon, periosteum, endoneurium | Loosely packed thin fibrils with more uniform diameter | Fibroblast, smooth muscle cells, Schwann cells, hepatocytes, mesenchymal precursor cells | Structural maintenance in expansile organs; wound healing, mediate attachments of tendon, ligament and periosteum to bone cortex |
Table 1. Adapted from Liu et al (1995)
Type III collagen is abundant in large blood vessels, skin, liver, spleen, and is found in small amounts in most tissues that contain type I collagen (table 1), but it is not present in bone (Williams et al 1997, Liu et al 1995). For example, the dermal collagen is constituted of 80% – 85% of type I collagen and 15% – 20% of type III collagen (Williams et al 1997). It is the earliest collagen, which is laid down in the healing process, and because its cross-links can be generated rapidly, they provide mechanical strength to the newly synthesized matrix. As the healing tissue gains in strength, the stronger type I fibres replaces type III fibres, if the injured tissue is tendon or ligament (Culav et al 1999). But, if the injured tissue is cartilage, type II fibres replace them.
Biosynthesis of fibrillar collagen
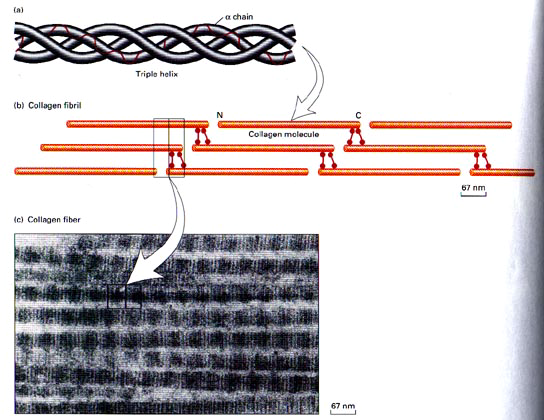
The initial transcript of the major fibrillar collagens (types I, II, and III) is secreted from the nucleus of the cell as interstitial collagen (fig.4). The biosynthesis of individual chains then occurs on the rough endoplasmic reticulum. Subsequently, the chains are folding in the individual triple chains to form the collagen molecule, which is named procollagen. Then, the collagen molecules move to the Golgi apparatus, by which they promoted and extruded from the fibroblast (Mayne 1997, Burgeson et al 1992).
Before fibril formation can occur, the procollagen molecules lose their noncollagenus extensions at their terminal regions and become negatively charged molecules named tropocollagen. Many molecules of the tropocollagen are arranged side by side to form the quarter-staggered fibrils. This configuration is visible in the electron microscope and characterized by distinct cross-band. The fibrils are stabilized by the formation of cross-links between individual molecules (Mayne 1997).
The fibrils join together to form fibres (fig.4), which in turn combine together to form bundles of collagen fibres The first can be seen only under the light microscope, while the bundles can be seen unaided (Culav et al 1999). This procedure occurs for the morphogenesis and remodelling of tendons. For the other tissue, it is poorly understood how collagen fibrils form and whether fibril formation is closely associated with the cell ( Mayne 1997). Fibrils may also be formed of more than one type of collagen (Culav et al 1999). For example “The banded cartilage collagen fibres are thought to contain types II and XI, and the noncartilage banded fibres contain mixture of types I, III, and V collagens.” (Burgeson et al 1992).
In tendons, the majority of fibres have parallel alignment. In ligaments, the collagen fibres are positioned in slightly less parallel arrangement. In skin, fibres are arranged in random orientation. Collagen plays an important role in attaching tendons and ligaments to bone. (Culav et al 1999)

b) Elastin.
The presence of rubber-like elastic fibres determines the elastic properties of tissues such as skin, large blood vessels, lung and large ligaments (fig.5). Elastic fibres are unstructured, in the sense that their molecular components are not assembled in the regular pattern. The major constituent of elastic fibres is elastin (Williams et al 1997).
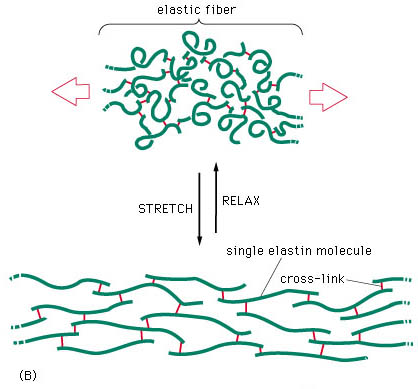
The elastin arrangement varies depending largely on the strength and direction of forces on the tissue. Elastic fibres consist of an elastic core with some microfibrils located mostly around the periphery (fig 6). The exact mechanism of extensibility is not clearly understood. However, the quantity of elastin found within the tissue is usually reflected by the amount of mechanical strain inflicted on it and the requirement for reversible deformation. Some tissue, such as tracheobronhial tree of the lung, walls of arteries, and dermis are rich in elastic fibres, but some others such as ligaments, contain relatively sparse amount of elastic fibres, except the ligamenta nuchae in cervical spine and ligamenta flava of the thoracic and lumbar spine (Culav et al 1999).
Biosynthesis of elastin
Elastin is synthesized by smooth muscle cells and to a lesser extent by fibroblasts, but how it is synthesized is unclear. After elastin is secreted, it undergoes extensive cross-linking reactions with other elastin monomers and creates an extended insoluble fabric that provides tissue with elastic properties (Williams et al 1997).
A major problem in adults is the lack of regeneration of functional elastic fibres. This results in the inability to restore normal function. Elastin, however, is synthesized by adult tissue in response to cyclic stretching, injury, and ultraviolet radiation. A number of disease states, including emphysema, also lead to synthesis of elastin. Adults, apparently, cannot rebuild the elastic fibre assembly mechanism, and function is not restored. Generally, the mechanisms of control of elastic fibres formation are unknown (Culav et al 1999).
2. Proteoglycans (PGs)
According to their composition in type of glycosaminoglycan chain (GAG), all proteoglycans are named: chondroitin sulphates 4 (CS A) and 6 (CS C), keratan sulphate (KS), dermatan sulphate (DS), heparan sulphate (HS), and hyaluronan (HA) (Williams 1995, pages 84-86). They hydrate, stabilize and fill the space of the extracellular matrix (Culav et al 1999).
The PGs are consisted of a core protein at which are covalently attached one or more sulphated glycoasaminoglycan (GAG) chain, except hyaluronan, which neither attached to a protein core, nor sulphated (Hukins 1990, Williams 1995). However, it is usually included into the PG group, because it is the most abundant of the GAGs, and it has an important role in connecting to the other PGs to form supramolecular compound structures (Culav et al 1999).
There are various GAG chains with generally specific cores to each of the PG types. The GAG chains consist of repeating disaccharide units. The type and the number of units largely define the properties of the PG. Because of their negative polarization, all GAGs have a propensity to attract ions, and create a high osmotic activity that results in the absorption of water from surrounding tissues (Hukins 1990). The amount of hydration depends on the number of GAGs chains (Culav et al 1999). According to Williams et al (1997), the polyanionic character of the proteoglycans and the resulting swelling pressure keep the collagen fibrils apart and award stiffness on the porous gel, which they create.
The percentage of GAG with in CT varies directly proportional to the mechanical load. The higher the compressive forces acting on the tissue, the larger content of PG in it (approximately 8%-10% of the dry weight of the tissue). Conversely, in tissues, which are exposed in tension loads such as tendons and ligaments, PGs are concentrated in relatively small proportion (approximately 0,2% of dry weight). (Culav et al 1999).

According to Gray’s Anatomy (Williams 1995, page 86), the proteoglycans are classified into:
• Small PGs, which consist of the globular protein as the head and one or two glycan chain as the tails.
• Large PGs, which contain one or two protein cores attached by 5-10 glycan chains and
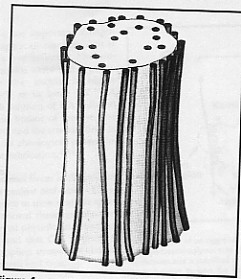
• Very large PGs, which are very complex molecules. They are consisted of 3 globular protein cores, which are bonded to hyaluronan. About 100 glycan chains are attached to each core (fig.7).
Culav et al (1999) divide the PGs in aggregating (large and very large) and nonaggregating (small) proteoglycans.
The small or nonaggregating PGs are attached at specific binding sites on the surface of collagen fibres and interact with them. The role of PGs in withstanding compression is limited, but they contribute to mechanical stability by their interaction with the collagen fibres. They are found in cartilage, disc, tendon, skin sclera, cornea.
The aggregating (large and very large) PGs are like the bristles of a bottlebrush, which fill the interfibrillar space. They are found in skin, sclera and tendon, and the very large ones are abundant in cartilage, disc and blood vessels. The large density of the aggregating PGs in cartilage and in the nucleus polposus of the intervertebral disc induces the movement of water into the matrix, resulting in the ability of these tissues to resist compressive forces (Culav et al 1999).
All PGs do not permit the neighbouring collagen fibres to been fused.
3. Glycoproteins.
Glycoproteins do not have mechanical functions, but they are important in stabilizing the surrounding matrix and linking the matrix to the cells. They regulate many functions of the cells such as producing changes in their shape, increasing their mobility and stimulating their proliferation. Among the best-known glycoproteins are fibronectin, tenascin, laminin, link protein, thrombospondin, osteropontin and fibromodulin.
Vessels and nerves of connective tissue
Generally, the blood supply of the connective tissue is poor, although some of the forms of the dense tissue, such as the dura mater and periosteum, have very rich blood support. Many blood vessels may pass through connective tissue en route for other tissues without supplying it (Williams 1995).
The lymphatic vessels are abundant in most types of the connective tissues, particularly beneath the skin. They are numerous in tendons, especially in their sheaths (Williams 1995).
Many kinds of nerve endings terminate in connective tissue and supply sensory innervation, which identify mechanical stresses, painful stimuli and thermal changes. Sympathetic innervation of the adipose tissue is important in controlling the metabolism of adipose cells, especially the breakdown of lipid (Williams 1995).
Embryology
Human development begins with fertilization of an ovum. But between this initial contact and birth a series of complicated changes occurs. This involves both differentiation and reorganization of cells to form the different tissues, organs and organ systems to create a viable individual.
Immediately after fertilization the zygote undergoes repeated mitotic divisions, resulting in a ball of cells. When the cell has divided into a mass of sixteen cells (Now called the Morula) it enters the uterus wall. Differentiation continues so cells within the Morula are labeled depending on their position and function.
Simplification of the cell structure can label these into three zones: The Ectoderm, Endoderm and Mesoderm (Palastanga et al 1994).
The Ectoderm gives rise to the skin and its derivatives and the central and peripheral nervous system. The Endoderm forms many epithelial tissues of the body which lines many major bodily systems, e.g. Digestive and respiratory tracts. The Mesoderm forms various structures including the dermis, circulatory system, bone and cartilage.
The Mesoderm also gives rise to many specialist cells including Mesenchymal cells. These cells differentiate and specialize again to form the soft tissues, bones, muscles and nerves of the body.

Adapted from Gray’s (1995), these structures of the neuromusculoskeletal system can be divided into three distinct subgroups of Mesenchymal cells:
Firstly paraxial mesenchymal cells are responsible for the development of the vertebrae, portions of the neurocranium and axial skeleton. All voluntary muscles throughout the head, trunk, limbs, and the dermis of the skin.
The neural crest mesenchymal cells are responsible for the development of many soft tissues throughout the head including nerves, viscera, ligaments, tendons, fascia, muscular and dermal connectives tissue and the meninges. The neural crest also patterns the development of the nerves, muscles and blood vessels in this location. Schwann cells too, which are key regulators of peripheral nerve development originate from the neural crest and differentiate into myelin and non-myelin forming cells.
Finally the somatopleuric mesenchymal cells give rise to the skeletal elements, ligaments, tendons, fascia, muscular and dermal connective tissue throughout the trunk and limbs. These specific mesenchymal cells also pattern the development of nerves, muscles and blood vessels in these locations.
Summary
Human cells undergo specific differentiation and specialization immediately after fertilization. Basic cell structure can be split into three zones; ectoderm, endoderm and mesoderm. Connective tissues arise from specialist cells within the mesoderm zone. These mesenchymal cells are further divided into three distinct subgroups; paraxial, neural crest and somatopleuric. All connective tissues found within the body arise from neural crest and somatopleuric cells.
Cells Differentiation
Movement of cells populations (morphogenesis) and differentiation involves cells and tissue interactions in the early embryo. The process is known as (Gray 1995)
induction.
It is a complicated stage where the induced tissue may change or both tissues involved also may change in various combinations.
In Human Function and Structure,(Luciano 1978) cells, in fact, multiply, migrate and specialize, forming layers which can fold, fuse, separate and elongate in order to shape the human body. Other cells, which have not undergone cell differentiation, are known as stem cells. Some other undifferentiated cells can remain and multiply in the various organs where, under an appropriate stimulus, may then go through a delayed differentiation into a specialized cell type.
At the stage of fertilization every cell in the growing embryo receives an identical set of genes some of which are either turned off or on (i.e., from messenger RNA and the corresponding protein). Cell differentiation depends on the above mechanism suggesting a quantitative as well as qualitative control of gene expression.
Two types of interactions are described (Gray 1995): Permissive and Instructive; in a permissive interaction the signal between the apposing tissue and the responding tissue does not influence the developmental pathway selected. The responding tissue has the intrinsic capacity to develop and to express this capacity it only needs the appropriate environmental conditions.
Permissive interactions are often more common in a later stage of the development where the pre-determined fate of some tissue is only maintained and stabilized by another.
An instructive interaction changes the cell type of the responding tissue.
Condensation, (Hall BK 1992) is described as the first cellular product following tissue interaction and they are attributed a pivotal role in initiation of the vertebrate skeleton in embryonic development and in the modification of skeletal morphology during evolution.
Therefore, particularly important are the interactions between Epithelial and Mesenchymal cells during embryonic development, which take place in a reciprocal manner. These spatial, temporal sequential interactions both permissive and instructive leading to the differentiation and morphogenesis of most tissues and organs are termed epigenetic cascades.
It is recognized (Rogers 1983) that different signaling patterns take part during cells differentiation, influenced by size, shape, spacing, orientation and activity of many thousands of cells, each behaving in a reproducible way that is appropriate to its position in the tissue or organ concerned. The simplest way in which one cell can influence another is the release of some signaling molecule into the extracellular fluid. However, there are other ways to make sure that communication is transmitted and the following classification is an example:
Direct cell-cell contact;
Cell adhesion molecules;
Extracellular matrix and their receptors;
Growth factors and their receptors;
Ultimately, Connective Tissue (CT) is therefore the result of specific processes that occur during early embryonic development (Watkins 1999).
Tendons, ligaments and aponeurosis for example are the result and a combination of few cells and large amount of non -cellular material named matrix, produced by the cells. It is, in fact, the matrix the element that gives the various types of CT a different structure and function also.
Generally speaking CT has two functions:
Mechanical support, concerned with providing strength or elasticity as in binding together the cells of the body, supporting various organs and holding them into place, providing shock absorption and flexible links between bones and transmitting muscle forces.
Intercellular exchange, concerned with the circulation of body fluids, hence homeostasis.
Nicky Hayden: thanks to my physiotherapist, Alfredo Dente

Nicky Hayden – Ducati Team, Jerez, MotoGP, 2013
“The small tweaks we did to the bike this morning were improvements, and after I was fifth in the warm-up with a decent pace, I was confident for the race. My start was okay, but I lost the gap when Bradl crashed in front of me and I wasn’t able to stay with Bautista and Crutchlow. Thanks to my physiotherapist, Alfredo Dente, and the Clinica Mobile, who helped me to race despite the problem with my wrist. With the adrenaline, the first fifteen laps weren’t much of a problem, but the last part wasn’t much fun. It was our best weekend so far this season, so hopefully we can build on it.” (motorcycledaily.com)
Motegi, MotoGP, 2012
“Thanks to the team for their work and to my physiotherapist, Freddie Dente, for helping me to manage the race with a fracture in my wrist.” (motorcycledaily.com)
Searching fisioterapista in Google News Italy
Today on Google News Italy the research fisioterapista (physiotherapist) will reveal in first position a press release about Alfredo Dente and his new physio clinic in Milan.

The Motorcycle Workout, askmen.com by Nicolas Stecher
In the article The Motorcycle Workout by Nicolas Stecher published on askmen.com
“Hard riding can definitely raise one’s heart rate to the same level as that in any hard fitness session. This happens for two reasons, the first being the physical effort a rider is required to expend when riding these bikes. The second is due to adrenaline, which is the most powerful heart stimulation anyone can get,” adds Alfredo Dente, official team physiotherapist at Ducati’s Super Aguri F1 MotoGP team. “But this is a situation which should not happen while cruising around on our public roads, since it would mean that speed limits and safety would definitely not be respected.”
Connective Tissue: Structure, injury and repair
Connective Tissue: Structure, injury and repair
Students & Student ID
Alfredo Dente
Robin Holland
Palina Karakasidou
Linda Khong
Jim Maskrey
Patrik Pedersen
Michael Wong
School/Department: Physiotherapy (Master of Manipulative Therapy)
Coordinator: DR Kathy Briffa
Date due: 8th May 2002 (Week 12)
Contents
- Embryology
- Classification of connective tissue
- Classification of general connective tissue
- Structure and function of ligament and tendon
- Mechanical properties of ligament and tendon
- Tendon injuries and contributing factors
- Ligamentous injuries and contributing factors
- Repair
- Clinical implications
- References




