Manutech is a new tool we chose to provide our client the best interaction between manual therapy and newest technology available.

Author: dente
Happy World Physical Therapy Day everyone… (8th September)
Today is World Physical Therapy Day, I am one and I am proud of being a Physical Therapist.
I have chosen, many years ago, to undergo specific studies and training to become a Physical Therapist although it was not totally clear to me what was involved with and what I was getting into.
It occurs to me often that a lot of people are not too aware of what we do, how we do it and why unless they get sick and that at a certain point, if they are lucky, they are referred to or somehow they turned to us for help.
We work closely with patients of all kinds and who are or have been affected by a desease or injury or simply recovering from a bad back pain.
Today I would like to think of all my collegues who tomorrow will go to work in a hospital or private clinic or nursing home, ultimately it’ s not important WHERE but what really should count is that one of us will be there to help a lady, an old man or a child to regain strength, self confidence and, why not, dignity.
Of course there is no magic, no tricks, no shortcuts only hard work and professional competence to motivate sombody to get back on their feet if possible or to manage at their best any possible outcome after a physical set back.
Tomorrow, like every other day, a Physical Therapist will help Doctors and Nurses in an intensive care unit, or will help a patient after a knee replacement, or even help another one to use a wheelchair or may be he will deal with somebody who has had a stroke and he/she is struggling to sit up straight or to use his/her arm and leg again.
The most important thing is that also thanks to Phisical Therapists many patients can be guided through a path of recovery and rehabilitation which can make their lives manageable or as it happens in many lucky cases almost back to normal although we (Physical Therapists) know that normality is sometimes a very abstract concept sometimes.
We are not special people with special skills on the contrary but we study, train and mostly try to keep up our skills and competence in order to do our job properly, sometimes in a hospital, private clinic or on a soccer pitch or racing track.
It doesn’ matter where who with and how, what should really count is that we always do it at our best with respect for ethics, dignity, pivacy and possibly with a smile.
Happy World Physical Therapy Day everyone.……
Alfredo Dente
Phisical Therapist and Manual Therapist
Knee pain, surgical and conservative approach at the Italian Academy of Osteopathic Medicine
Alfredo Dente will be teaching at the Italian Academy of Osteopathic Medicine (aimo-osteopatia.it) in Saronno on the 28th and 29th of November.
The course theme will be on Knee pain, surgical and conservative approach.
Alfredo will bring his clinical experience on the subject and will support a productive discussion with the other participants:
- Dr Robero Abba (Orthopaedic Consultant)
- Dr Emanuele Ungaro (orthopaedic surgeon and Osteopath)
- Dr Renato Varinelli (Orthopaedic Surgeon)
- Lorenza Dacò (Personal Trainer)
aimo-osteopatia.it/it/postgraduate/la-gonalgia-expert-opinions-pareri-e-approcci-a-confronto/145
Amazing performance in wet condition by #VR46 @ValeYellow46 at @SilverstoneUK @MotoGP
Amazing performance in wet condition by VR46 at Silverstone. Good job also for Petrucci and Dovizioso
— Freddyphysio (@alfredodente) 31 Agosto 2015
References
Alfredson H, Pietilä T, Jonsson P and Ronny L (1998): Heavy-Load Eccentric Calf Muscle Training For the Treatment of Chronic Achilles Tendinosis. The American Journal of Sports Medicine 26: 360-366.
Alfredson H and Lorentzon R (2000): Chronic Achilles Tendinosis Recommendations for treatment and Prevention. Sports Medicine 29: 135-146.
Archambault DM, Wiley JP and Bray RC (1995): Exercise loading to tendon and the development of overuse injuries, a review of current literature. Sports Medicine 20: 77-89.
Binkley J (1989): Overview of ligament and tendon structure and mechanics: implications for clinical practice. Physiotherapy Canada 41: 24 – 31.
Boorman RS, Shrieve NG and Frank CB (1998): Immbolization increases the vulnerability of rabbit medial collateral ligament autografts to creep. Journal of Orthopaedic Research 16: 682 – 689
Burgeson RE and Nimni ME (1992): Collagen Types. Molecular Structure and Tissue Distribution. Clinical Orthopaedics and Related Research 282: 250-273.
Culav EM, Clark CH and Merrilees MJ (1999): Connective tissue: Matrix composition and its relevance to Physical Therapy. Physical Therapy 79: 308-319.
Frank C, Shrive N, Hiraoka H, Nakamura N, Kaneda Y and Hart D (1999): Optimisation
of the biology of soft tissue repair. Journal of Science and Medicine in Sport 2: 190 – 210.
Frank CB (1996): Ligament healing: current knowledge and clinical applications. Journal
of American Academy of Orthopaedic Surgeons 4: 74 – 83.
Frankel V and Nordin M (1980): Basic biomechanics of the skeletal system. Philadelphia: Lea & Febiger.
Gray’s Anatomy (38th ed.) (1995): London: Churchill Livingstone.
Gum L S, and Reddy G K (1997): Combined Ultrasound, Electrical Stimulation, and Laser Promote Collagen Synthesis with Moderate Changes in Tendon Biomechanics. American Journal of Physical Medicine & Rehabilitation 76: 288-296
Hall BK, Miyake T (1992): The Membranous skeleton: the role of cell condensations in vertebrate skeletogenesis. Anatomy and Embryology. Review, Tutorial 186(2):107-24.
Hayman J and Rodeo SA (2000): Injury and repair of tendons and ligaments. Physical
Medicine and Rehabilitation Clinics of North America 11: 267 – 288.
Hildebrand KA and Frank CB (1998): Scar formation and ligament healing. Canadian
Journal of Surgery 41: 425 – 429.
Hukins D W L (Ed) (1990): Connective Tissue Matrix (Part Two). Hampshire: The Macmillan Press.
Hukins DW (1990): Dynamic Aspects of Connective Tissue Structure and Function. London: Macmillan Press.
Huijbregts PA and Smith SE (1999): Tendon Injury: A Review. The Journal of Manual
and Manipulative Therapy 7: 71-80.
Hunter G (1994): Specific Soft Tissue Mobilisation in the Treatment of Soft tissue Lesions. Physiotherapy 80: 15-20.
Hyman J and Rodeo SA (2000): Injury and Repair of Tendons and Ligaments. Physical Medicine and Rehabilitation Clinics of North America 11: 267-288.
Jozsa L and Kannus P (1997): Human Tendons: anatomy,physiology and pathology: Human Kinetics.
Kennedy J, Hawkins R, Willis R and Danylchuck K (1976): Tension studies of human knee ligaments. Yield point, ultimate failure and disruption of the cruciate and tibial collateral ligaments. The Journal of Bone and Joint Surgery 58: 350-355.
Leadbetter WB (1992): Cell-Matrix Response in Tendon Injury. Clinics in Sports
Medicine volume 11: 533 – 578.
Liu SH, Yang R-S, Al-Shaikh R and Lane JM (1995): Collagen in tendon, ligament, and
bone healing: a current review. Clinical Orthopaedics and Related Research 318: 265 –
278.
Luciano, Dorothy S.(1978): Human Function and Structure: New York: McGraw-Hill, Inc.
Mayne R (1997): Arthritis and Allied Conditions. A Textbook of Reumatology (13th ed). Baltimore: Williams & Wilkins.
Nigg B and Herzog W (1999): Biomechanics of the musculoskeletal system. (2nd ed.) New York: John Wiley & Sons.
Noyes F (1977): Functional properties of knee ligaments and alterations induced by immobilisation. Clinical Orthopaedics 123: 210-242.
Noyes F and Grood E (1976): The strength of the anterior cruciate ligament in humans and rhesus monkeys. The Journal of Bone and Joint Surgery 58: 1074-1082.
Noyes F, Torvik P and Hyde W (1974): Biomechanics of ligament II. An analysis of immobilisation, exercise and reconditioning effects in primates. The Journal of Bone and Joint Surgery 56:
O’Brien M (1992): Functional anatomy and physiology of tendons. Clinics in Sports
Medicine 11: 505-520.
Palastanga N, Field D and Soames R (1994): Anatomy and Human Movement, Structure And Function (2nd ed.) Oxford: Butterworth Heinemann
Panjabi M and White IA (2001): Biomechanics in the musculoskeletal system. New York: Churchill Livingstone.
Reid D (1992): Sports injury assessment and rehabilitation. New York: Churchill Livingstone.
Reid DC (1992): Sports Injury Assessment and Rehabilitation. New York: Churchill & Livingstone.
Rogers, Andrew W. (1983): An Introduction to Histology and Cell Biology: London: Academic Press Inc.
Rundgren A (1974): Physical properties of connective tissue as influenced by single and repeated pregnancies in the rat. Acta Physiol Scandanavia Suppl: 417.
Sandrey MA (2000): Effects of acute and chronic pathomechanics on the normal
histology and biomechanics of tendons:a review. Journal of Sport Rehabilitation 9: 339-
352.
Schultz R, Miller D, Kerr C and Micheli L (1984): Mechanoreceptors in human cruciate ligaments. The Journal of Bone and Joint Surgery 66A (7): 1072-1076.
Watkins James, (1999): Structure and Function of the Musculoskeletal System.
Williams CJ, Vandenberg P and Prockop DJ (1997): Textbook of Rheumatology (5th ed). Philadelphia: W.B. Saunders Company.
Williams PL, Bannister LH, Berry MM, Collins P, Dyson M, Dussek JE and MWJ Ferguson (Eds) (1995). Gray’s Anatomy (38th ed). New York, Churchill Livingston: 75-90.
Wilson AM and Goodship AE (1994): Exercise-induced hyperthermia as a possible mechanism of tendon degeneration. Journal of Biomechanics 27: 899-905.
Woo S, Gomez M and Inoue M (1983): Measurement of mechanical properties of ligament substance from a bone-ligament-bone preparation. Journal of Orthopaedic Research 1: 22-29.
Woo S, Gomez M and Sites T (1987): The biomechanical and morphological changes in the medial collateral ligament of the rabbit after immobilization and remobilization. The Journal of Bone and Joint Surgery 69A: 1200-1211.
Clinical implications
Which Phase?
The therapist should always determine the phase of the inflammatory process before starting treatment. Acute traumatic injuries often present with a clear time of onset, but with overuse injuries, because of the insidious onset, it is more difficult to identify the current phase. Continuous reinjury as is common with overuse will repeatedly set back the progression of the inflammation phase, and therefore it is impossible to tell when the inflammatory process started. Because of this the therapist should always, unless proven otherwise assume that it is in the vascular inflammatory phase (Hujibregts and Smith 1999). Treatment of acute and chronic ligament/tendon injuries can be confusing because of the fine balance between adequate rest and preventing atrophy of surrounding muscles. This has led to the development of very detailed exercise programs of for example ACL, Achilles- and Patella tendon (Hyman and Rodeo 2000).
Healing of connective tissue
During the inflammation phase the tensile strength is dependant upon fibrin, which is very fragile, and tension could easily disrupt it. In order to control it is essential to limit the amount of post-injury oedema using ice, elevation compression. Other forms of management are NSAID´s and modified rest. Ultrasound and laser has shown to accelerate the inflammation process (Hunter 1994, Reid 1992).
In the regeneration phase it is important to maximize strength, the collagen fibres should relate to the undamaged tissue. Careful tensioning during this phase promotes correct alignment by remodelling the collagen fibres. The rate of increase in wound strength is proportional to the amount of collagen laid down (Hunter 1994).
Management in the remodelling phase is to minimize immobilization, balance functional stresses with increasing tissue strength, re-establish ROM and enhance proprioception (Reid 1992).
Prevention of scar tissue and contraction and promoting the correct orientation of collagen fibres is essential for the best functional outcome. The wound should be subjected to tension on a regular basis to promote extensibility of the scar tissue (Hunter 1994).
Modalities and pharmacology
There has been a great deal of research on the ability to influence the basic biology of tendon healing using pharmacological and physical modalities. Low intensity laser beam (photo stimulation) and electric stimulation has been shown to enhance tissue healing the research showed an increased total collagen production, but there were fewer cross-links per collagen molecules, the mechanism remains unclear. It is suggested that it promotes nucleic acid synthesis and cell division of human fibroblasts. Ultrasound has been reported to improve scar maturation and to decrease inflammatory cell infiltration into healing flexor tendon (Hyman and Rodeo 2000). A combination of ultrasound, electrical stimulation and laser stimulation was found to increase collagen synthesis and tensile strength in healing rabbit’s tendons (Gum and Reddy 1997).
Cold is useful in the immediate care of an acute tendon injury to decrease inflammation, oedema and pain. Heat has been shown to increase chemical activity, metabolic rate and tissue extensibility and decrease muscle spasm, pain and ischemia. Heat should not be used in the acute phase of the inflammation (Józsa and Kannus 1997) .
Various growth factors have been found to effect cell proliferation, matrix synthesis, and cell migration in intrasynnovial and extrasynnovial tendons, the most promising are (TGF, IGF-1 and PDGF). Ligaments treated with PDGF, IGF-1 and FGF had increases in strength, stiffness and breaking energy (Hyman and Rodeo 2000).
The current opinion on the use of NSAID´s in acute tendon strains and partial tendon ruptures should be administrated during the inflammation and proliferation phase (Józsa and Kannus 1997).
Tensile loading/stress
In the early stages of rehabilitation it is of great importance that the injured tissue is well protected. Maintaining the blood supply is critical to prevent necrosis of the collagen, and this may be a problem in areas with compromised vascularity because of friction, compression or torsion, for example achilles and supraspinatus tendon. It is also important to have a regime of progressive tensile stress, which will help the fibrillar alignment, appropriate cross-linking and normal mechanical properties, during subsequent phases. Research shows that exercise has a positive effect on mechanical and morphological characteristics of the injured tissue. There is a problem with applying a progressive regimen of tensile force to strengthen the tissue without reinjuring it (Hujibregts and Smith 1999).
Deep friction can in early phases enhance the mobilisation of tissue fluid, and in later stages stimulate fibre orientation, prevent cross linking and adhesions and increase blood flow.
Stretching should be used with increased tissue temperature and slow prolonged stretching to gain a permanent increase in ROM (Reid 1992).
Ligaments
The rehabilitation of ligaments depends on if the joint is stable or not, unstable joints may need bracing techniques or surgery. Injured ligaments have abnormal proprioception, which makes the proprioceptive training another factor to include in the rehabilitations program.
Joint position sense (JPS) in ACL deficient knees has been described as impaired, although knee stability can improve with exercise therapy, there may be no improvement in JPS. The role of JPS in the stability of ACL deficient knees remains unclear (Hyman and Rodeo 2000).
Heavy load eccentric calf muscle training for chronic achilles tendinosis
A study on eccentric training was conducted with 15 middle-aged recreational athletes, who had the diagnosis chronic achilles tendinosis (degenerative changes) and where selected for surgical treatment. They also had a control group with the same diagnostic criteria that underwent surgical treatment. The training group followed an eccentric training program 2 times a day for 12 weeks. The program included eccentric calf muscle training with both the knee extended and flexed (3×15 for each exercise). The patients were told to go ahead with the exercise even if they experienced pain. When they could perform the exercise without pain they were instructed to increase the load.
After 12 weeks the training group were all back to penury level. The control group were all back to pre-injury level after 6 months.
Possible explanations may be either the effect of stretching with a lengthening of the muscle-tendon unit, and consequently less strain during ankle joint motion. Or loading within muscle tendon unit with hypertrophy and increased tensile strength in the tendon, thus remodelling is induced from eccentric loading (Alfredson and Lorentzon 2000, Alfredson et al 1998).
Ligament Repair and its clinical relevance
Ligament or tendon injury occurs when the load acting on the tissue is over its own capacity. When the load is over phase III in the above-mentioned stress-strain curve, tissue failure occurs. As soon as the ligament starts to heal up after the injury, usual healing process will proceed namely: 1. inflammatory; 2. cellular and matrix proliferation (regeneration); and 3. remodeling.(Hildebrand and Frank 1998, Reid 1992)
In the following, changes in the tissues structures as well as their mechanical properties during the healing processes will be discussed.
A. Healing response within tendon and ligament toward injury
I. Inflammatory phase
At the cellular level, alteration of structural makeup starts.
• Formation of fibrin clot (Hildebrand and Frank 1998, Reid 1992).
• Fibroblasts proliferation (Hayman and Rodeo 2000, Liu et al 1995, Reid 1992).
• Ligament: High Type III collagen (loosely packed thin fibrils), low Type I collagen (densely packed thick fibrils)
Tendon: Similar amount of Type I & III production (Liu et al 1995).
II. Regeneration
At cellular level:
• Type I collagen fibrins bridge up the gap between the torn ends (Hildebrand and Frank 1998).
• Spare collagen framework formed (Reid 1992).
• Formation of scar with Type I collagen comprises large percentage of scar tissue (Frank 1996, Liu et al 1995).
• Fibroblast remains predominant cell.
III. Remodeling
1. At cellular level:
• There is an increase of collagen density & cross link, alignment of collagen fibers in the axis of ligament (Hayman and Rodeo 2000, Liu et al 1995).
• Histological normal ligament after 7 months, mechanical properties still not fully returned to normal state.
• Still high type III collagen percentage in repaired ligament (Liu et al 1995)
• Alignment of collagen fibers is longitudinally along lines of stress in tendon, minimal histological difference from normal tendon after 20th week.
2. Mechanical properties of an healed injured tendon and ligament
• The tensile strength shows dramatic reduction (Reid 1992).
• Ligamentous strength after repair is in the region of 60 to 70% normal after 6 weeks of healing (Reid 1992).
• It takes up 3 months before 80% of the original strength is acquired (Reid 1992).
• Intra-articular ligaments usually gain tensile strength more slowly, 3 months 50% of normal strength & 6 months 70% functional strength. (Reid 1992).
• Strength and stiffness restore to 40 – 90% of normal values in animal studies, only about 30 – 70% of the material strength has returned (Frank 1996).
• Viscoelastic properties appear to return to 70 – 90% of normal values (Frank 1996).
• Ligament (using MCL) heals structurally 70 – 80% of the strength and structural stiffness of a normal MCL. The scar reaches a maximum of only about 30% of normal MCL strength, even after months to years of healing (Frank et al 1999, Frank 1996).
3. Formation of scar tissue
• Scar development: inflammation and formation of granulation tissue, scar proliferation and scar remodeling (Frank 1996).
• Comparison between ligament scar and normal ligament properties (Table 1.)
| Normal ligament | Ligament Scar |
| Collagen aligned | Collagen disorganized |
| Collagen densely packed | Defects between collagen fibers |
| Large collagen fibrils | Small collagen fibrils |
| Mature fiber cross-links | Immature cross-links |
| Primarily collagen type I (< 10% type III) | More collagen type III |
| Small proteoglycans | Some large proteoglycans |
| Other components minor | Excesses of other components |
| Cell metabolism low | Cell metabolism high |
| Low cell density | Increased cell density |
| Low vascularity | Increased vascularity |
• 3 major reasons for scar weakness – “flaws”, smaller than normal collagen fibril sizes and abnormal collagen cross-linking (Frank et al 1999, Hayman and Rodeo 2000).
• Normal ligaments Densely packed nearly parallel arrangements of a range of sizes of collagen fibers, cross-links are stable and resistant to breakdown. However the scar tissue has poorer cross-links and needs months to realign and abnormal collagen cross-linking (Frank et al 1999, Hayman and Rodeo 2000).
B. Effects of Immobilization and Mobilization
• Immobilization protects some ligament repairs grossly, causes isolated ligament scars to be less stiff and significantly less strong than scars in the joint that have been allowed to move, decreases ligament strength, has potential minimization of scar length which leads to ligament laxity (Frank 1996).
• Study in rabbit’s ACL showed changes in the shape and intracellular structure of fibroblasts from the ligament after immobilization, ligament switches progressively from an anabolic to a more catabolic state (Boorman et al 1998).
• Mobilization with some degree of mechanical load appears to be essential to the normal maturation and maintenance of the structural and mechanical properties of ligaments (Boorman et al 1998).
• Mobilization with controlled movement has been shown to improve scar stiffness and strength without compromising scar length, shown to stimulate collagen synthesis, matrix remodeling, production of better quality scar when compared with those produced with immobilization, production of increased scar mass to resist the tensile stresses involved (Frank 1996).
• Movement creates tension that increases fibroblast proliferation, migration and collagen synthesis, aligns the fibroblast & collagen fibrils parallel to the direction of the force. Therefore a healing ligament will have a high tensile strength (Liu et al 1995).
• In tendon, passive mobilization can prevent adhesion between the sheath and the healing tendon that restricts motion.
• In conclusion, mechanical stimulus (mobilization) has a significant affect on ligament and tendon structure.
Overview on management of tendon / ligament injuries
Grade I injury
| Signs | Management |
| – Minimal loss of structural integrity | – Minimal functional loss |
| – No abnormal motion | – Early return to training – some protection may be necessary |
| – Little or no swelling | |
| – Localized tenderness | |
| – Minimal bruising |
Grade II injury
| Signs | Management |
| – Significant structural weakening | – Tendency to recurrence |
| – Some abnormal motion | – Need protection from risk of further injury |
| – Solid end feel to stress | – May need modified immobilization |
| – More bruising and swelling | – May stretch out further with time |
| – Often associated hemarthrosis & effusion | – Dramatic reduction of tensile strength, a ware of the dangers of unduly stretching healing structures at this point. |
Grade III injury (complete rupture)
| Signs | Management |
| – Loss of structural integrity | – Needs prolonged protection |
| – Marked abnormal motion | – Surgery may be considered |
| – Significant bruising | – Often permanent functional instability |
| – Hemarthrosis |
Ligamentous Injuries: Classification
First category
– minimum tearing of a few fibres
– some pain
– little loss in structural integrity, no joint instability felt clinically
Second category
– moderate tearing of collagen fibres
– severe pain
– some loss of structural integrity, joint instability can be detected clinically
Third category
– most of the collagen fibres have ruptured, near complete tear
– severe pain
– joint is completely unstable
–
Factors affecting the Mechanical Properties of Ligament
Factor: Maturation and Aging
During maturation (up to 20 years of age),
-the number and quality of cross-links with the collagen molecules increases
-collagen fibril diameter increases
There is an overall increased tensile strength and stiffness of the ligament. After maturation, with aging, there is a gradual decrease in strength and stiffness.
In one study, the ACL was found to exhibit age-related changes. The young (16-26 years) adults’ are considered to be 3 times stronger than those of older adults (48-86 years), 1.7 times for the elastic modulus i.e. stiffer and 1.5 times for failure strain i.e. ultimate load (Panjabi and White, 2001).
Factor: Activity, Exercise and Disuse
Activity/ Exercise
Ligaments appear to remodel and become stronger and stiffer when there is mechanical demand on them (Noyes et al, 1974). Physical training improves the tensile strength and also the ligament-bone interface (Woo et al, 1983). Daily activity is sufficient to maintain 80-90% of a ligament’s mechanical potential. It has been found that injured ligaments that are exercised have significantly better physical and biomechanical properties than those not exercise (Nigg and Herzog, 1999).
Disuse/Immobilisation
In contrast, immobilization and disuse, decreases the tensile strength and stiffness of ligaments. In a study by Woo et al(1987) on rabbit knee, after 9 weeks of immobilization there was a corresponding 69% decrease in ultimate load and an 82 % decrease in energy to failure. Biomechanically, the water content decreases, collagen mass is reduced whilst the number of cross-links is increased and the ligament becomes stiffer.
Immobilization has a more immediate and substantial effect compared to effects of exercise. Upon remobilization, the effects of immobilization are reversible but considerable time will be needed to regain the former strength and stiffness. Noyes (1977) experimented 8-weeks immobilization on animal ligaments and found that 12 months of re-conditioning was necessary before it was comparable to initial values.
Factor: Pregnancy and Postpartum Period
Rundgren (1974) reported that the decreased stiffness of tendons and ligaments during early postpartum was restored later.
Factor: Temperature
Generally, ligaments become stiffer with cold and have increased extensibility with heat.
Factor: Ligament differentiation- Properties
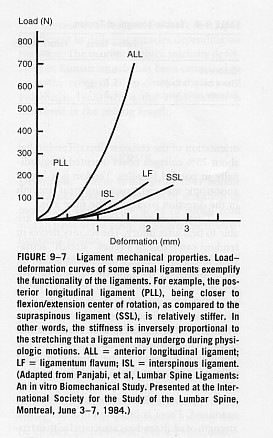
-Though ligaments follow a general stress-strain curve, the diagram below demonstrates that different ligaments exhibit different degree of stiffness and elasticity, hence different failure properties to injury. Posterior Longitudinal ligament is the stiffest and the most flexible ligaments are interspinous and supraspinous ligaments (Panjabi and White, 2001).
-Differences exist between ACL and MCL in their biologic responses during its midsubstance injury. MCL heals quickly and predictably even without repair whilst ACL fails to establish a significant healing response even if direct repair is done. There are ultrastructural differences. The cells of MCL are similar to those of fibroblasts (synthesis of collagen) but the ACL resemble fibrocartilage cells.
Factor: Diabetes Mellitus
Diabetes Mellitus is a disturbance of the normal insulin mechanism. When compared to nondiabetics, diabetics show a higher rate of tendon contracture (29% vs 9%), tenosynovitis (59% vs 7%), joint stiffness (40% vs 9%) and capsulities (16 % vs 1 %).
Tendon Injury: Sites
• Osteotendinous junction (trauma/overuse or disease)
• Mid tendon (often occurs in degenerative tendons – tendinosis)
• Musculotendinous junction (vulnerable point, common site of tendinitis)
Tensile strength of a healthy tendon is normally more than twice the strength of its muscle and stronger than compact bone. A tendon with a cross sectional area of 1cm² is capable of supporting a weight of 500 – 1000kg (Jozsa and Kannus 1997). Therefore unless the tendon is unhealthy it will normally breakdown at the MTJ. The MTJ has a delicate ultrastructure and is often the weakest point of the muscle-tendon unit making it more susceptible to injury (Jozsa and Kannus 1997).
Tendon Injury Patterns
• Acute
• Chronic
Acute tendon strains
Excessive loads greater than 4 -10% of original length or direct contusion will cause acute injury to tendons. Under normal physiological circumstances tendons function in the toe and linear region of the stress-strain curve. Loads greater than this will cause damage most often at the MTJ. Muscles that cross two joints are predisposed – hamstring, rectus femoris, gastroc. This normally occurs when lengthening of the muscle combines with eccentric contraction.
Chronic/overuse tendon injury
Repeated loading has been shown to cause overuse tendon injuries when the destructive forces of applied stress exceed the reparative process. This occurs when the tendon is strained repeatedly to 4 – 8% of its original length until it is unable to endure further tension, where upon injury occurs (Hyman and Rodeo 2000).
This causes micro and macroscopic injury to collagen fibrils, noncollagenous matrix, and microvasculature causing inflammation. It is currently believed that the earliest pathophysiological changes occur in the paratendon (surrounding the tendon) causing paratendinitis. Continued microtrauma whilst in the inflammatory stage will lead to proliferation of synovial cells, fibroblasts and capillaries leading to fibrosis and thickening of the paratendon. Thus, tendonitis and paratendinitis are the earliest clinical manifestations of overuse tendon injury. If the damage progresses, tendinosis, partial tears and complete rupture may ensue (Jozsa and Kannus 1997).
Classification of Injury
Paratendinosis is inflammation of the paratendon only, either lined by synovium or not. It can be acute or chronic. Signs and symptoms: swelling, pain crepitus, local tenderness, warmth, and dysfunction.
Tendinitis is symptomatic degeneration of the tendon with vascular disruption and inflammatory repair response. Symptoms are inflammatory and proportional to the vascular disruption. It can be acute, subacute or chronic.
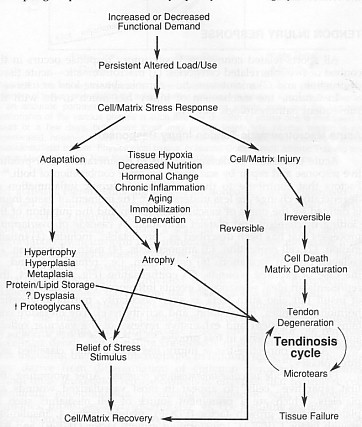
Tendinosis is when the tendon undergoes intratendinous degeneration due to cell atrophy, affecting its mechanical properties. The result of the pathological changes is decreased tensile strength that predisposes the tendon to injury. It is often asymptomatic since it is non inflammatory. It can be defined as “a change in tissue from a higher to a lower or less functionally active form”(Leadbetter 1992).
(Leadbetter 1992) illustrating Achilles paratendonitis +/- tendinosis
Classification of Tendon injuries and disorders
intratendinous degeneration due to atrophy (aging, microtrauma, vascular) can be nonsymptomaticsymptomatic degeneration of tendon with vascular disruption and inflammatory repair response
| NEW | OLD | DEFINITION |
| Paratendinitis | Tenosynovitis Tenovaginitis Peritendinitis |
inflammation of only the paratendon either lined by synovium or not. |
| Paratendinitis with tendinosis | Tendinitis | paratenon inflammation associated with intratendinosis degeneration |
| Tendinosis | Tendinitis | intratendinous degeneration due to atrophy (aging, microtrauma, vascular) can be nonsymptomatic |
| Tendinitis: | Tendon strain/tear acute, subacute chronic | symptomatic degeneration of tendon with vascular disruption and inflammatory repair response |
Adapted from (Leadbetter 1992)
Factors contributing to tendon injury (extrinsic/intrinsic)
• Excessive or Repeated loads – causes:
Poor biomechanics, joint laxity, leg length discrepancy – will alter direction, speed and size of force. e.g. foot hyperpronation has been linked to Achilles tendon injuries.
Muscle imbalances – weak muscle, tight muscle, poor timing of muscles can alter force distribution leading to strains.
Muscle fatigue, poor flexibility, – may increase load on tendons
Overweight – increased load
Training errors, poor equipment – e.g. prolonged exercise duration, could increase load
• Vascularity – if the tendon has a reduced blood supply (hypovascularity) it can lead to tissue hypoxia resulting in tendon cell atrophy contributing to tendon degeneration that results in tendinosis.
Hypovascularity has been linked most frequently to explain hypoxic degenerative tendon changes (O’Brien 1992). (Archambault et al 1995) reported that blood supply is irregular to the TA tendon mid-portion and at sites of twisting or around bony prominences. This study reported decreased vascularity in the TA tendon 4cm proximal to its calcaneal insertion. He suggested the cause was due to intermittent ischaemia during exercise – with subsequent reperfusion – leading to influx of oxygen derived free radical – these are highly reactive molecules that have a role in phagocytosis leading to tissue self destruction/degeneration.
Secondly, hypovascularity is linked to calcifying tendinopathy – persistent hypoxia may transform tenoblasts into chondroblasts that function anaerobically. Results in calcium deposition within the tendon.
Thirdly, regions of the tendon with poor vascularity will be less likely to cool over heated tissue caused by muscle activity, leading to exercise induced hyperthermia. This study reported that the intratendinous temperature of superficial digital flexor tendons of exercising horses (43 – 45º) exceeds the temperature at which fibroblast cell death occurs (42.5º). Thus, avascular regions of tendons may lack the ability to cool down the tissue leading to hyperthermic damage during exercise (Wilson and Goodship 1994).
• Genetic disorders/disease/infection – genetic collagen disorders like Ehlers-Danlos syndrome results in less cross-linking leading to weaker tendons. Rheumatoid diseases causes gross structural changes to tendons. Penetrating wounds can lead to tendon infections.
• Aging – results in stiffer, less compliant, weaker tendons with a reduced repair ability increasing their susceptibility to injury. This is due to decreased elastin, water and proteoglycan content, reduced collagen resynthesis and reduced vascularity and tenoblast activity (Hyman and Rodeo 2000).
• Endocrine factors – Diabetes Mellitus results in a compromise to tendinous microcirculation which may lead to tissue anoxia, cell necrosis thus predisposing a diabetic tendon to chronic paratendinitis or tendinosis (Jozsa and Kannus 1997). Hormonal changes in women can reduce the levels of estrogen, which has been linked to decreased collagen production.
• Nutritional deficiencies of cofactors (vitamin A, C and copper) important in collagen synthesis and cross-linking may affect tensile strength of tendons.
• Inactivity/Immobilization – (Jozsa and Kannus 1997) studied the effect of immobility on a human MTJ. Results showed the contact area between muscle cells and tendon collagen fibrils decreased to almost 50% in 3 weeks. There was also an increase in the amount of type III collagen at the MTJ, which is weaker than type 1 and a decrease in the amount of tenascin, an adhesive protein found in the MTJ. The overall effect will be to decrease tensile strength.
• Medication – corticosteroids (oral/injections) and certain antibiotics (flouroquinolone) can lead to pathology of the cell matrix. Excessive use of corticosteroid injections has been linked to tendon rupture (Jozsa and Kannus 1997). This may be due to its anti-inflammatory effect, as the injection reduces the pain, the patient will resume normal activity on the weak, degenerative tendon which could result in rupture.
• Skeletal maturity – osteotendonopathies like Osgood-Schlatters disease at the tibial tuberosity or Severe’s disease at the calcaneus can occur in the immature child due to tensile overload at the apophysis. Avulsions of small areas of the ossification center are found.
Mechanical Properties of Ligaments and Tendons
There are three major types of behaviour characteristic of viscoelasticity. Firstly creep, this is increasing deformation under constant load in the elastic portion of the stress-strain curve (Sandrey 2000). So that with a constant load, collagen length will increase over time. (Huijbregts and Smith 1999).
There are 3 major regions of the stress-strain curve that are of significance:

I. Toe region:
As the load increases so does the recruitment of collagen fibres causing them to ‘uncrimp’. This occurs when collagen is stretched to approximately 2% of its original length and returns to normal length when the force is removed, thus it is within its physiological range. It is characterized by relatively low stiffness. There is a non-linear relationship on the stress-strain curve at this stage.
II. Linear region (Elastic phase):
As the collagen fibrils become gradually uncrimped, the fibril itself is being stretched. There now becomes a linear relationship between deformation and load, as the tissue becomes relatively stiffer. This occurs when collagen is stretched to 2-4% of its original length and returns to its original geometric shape. The tissue is said to be elastic.
III. Yield and Failure region (Plastic phase):
The continued increase in load past 4% causes microfailure to the fibrils and damage to cross-links. It results in a plateau effect on the curve: this point represents the ultimate tensile strength of the tendon and is termed the ‘yield point’. The yielding of fibres occurs when the deformation is approximately 4-10% of the resting length. Stiffness is reduced and the fibrils do not return to normal length on release, the tissue then becomes ‘viscous’. This is known as ‘plastic’ deformation.
Finally, complete failure occurs as the ligament/tendon ruptures. Obviously this is a non-physiological range and there would be an inability to support load or function (Huijbregts and Smith 1999, Jozsa and Kannus 1997, Reid 1992).
Collagen demonstrates various mechanical and physical properties in response to load and deformation to allow it to withstand high tensile stresses. The point between the elastic and plastic region is where gross integrity is disrupted.
Viscoelasticity
Tendons and ligament are viscoelastic materials and display sensitivity to different strain rates. Viscoelasticity indicates collagen’s property of demonstrating time dependent and variable elastic behaviour. Thus, the relationship between stress and strain is not constant but depends on the time of displacement or load.
There are three major types of behaviour characteristic of viscoelasticity. Firstly creep, this is increasing deformation under constant load in the elastic portion of the stress-strain curve (Sandrey 2000). So that with a constant load, collagen length will increase over time. (Huijbregts and Smith 1999).
The second behaviour characteristic is force relaxation. This can be defined as the decrease in the amount of force required to maintain a set amount of deformation over time (linear region of curve). This occurs because the tissue relaxes (stress relaxation).
The third characteristic is hysteresis or energy dissipation. This means that if a viscoelastic material is loaded and unloaded, the loading curve will not follow the loading curve. The difference between the two curves represents the amount of energy that is dissipated or lost during loading. It refers to the amount of relaxation the tissue demonstrates during single cycle of deformation and relaxation. It is therefore an indication of the viscous properties of the tissue.